- Print:

Stryker receives US FDA HDE approval for the Neuroform Atlas™ Stent System
Kalamazoo, Michigan – November 9, 2017
 Stryker Corporation announced today that the U.S. Food and Drug Administration has approved the Neuroform Atlas™ Stent System for marketing under a humanitarian device exemption (HDE). The device is to be utilized in conjunction with neurovascular embolic coils for the treatment of wide neck, intracranial, saccular aneurysms and allows the company to begin U.S. commercialization efforts immediately.
Stryker Corporation announced today that the U.S. Food and Drug Administration has approved the Neuroform Atlas™ Stent System for marketing under a humanitarian device exemption (HDE). The device is to be utilized in conjunction with neurovascular embolic coils for the treatment of wide neck, intracranial, saccular aneurysms and allows the company to begin U.S. commercialization efforts immediately.
“The hybrid cell stent design of Neuroform Atlas is designed to improve wall apposition, ease of use, deployment accuracy, and catheter re-entry in even the most challenging cases,” said Dr. Osama O Zaidat, Director of the Neuroscience and Stroke Center at Mercy Hospital in Toledo, Ohio, and Co-Principal Investigator of the U.S. Neuroform Atlas investigational trial. “The Atlas design may improve patient care by facilitating the treatment of wide neck aneurysms in tortuous and more complex anatomies.”
“The ability to navigate distal anatomy within the brain using the lowest profile delivery on the U.S. market is a significant advantage to physicians,” said Dr. Brian Jankowitz, Director of the NeuroEndovascular Fellowship program at the University of Pittsburg Medical Center and Co-Principal Investigator of the study. “Atlas opens up treatment options for a new segment of patients that would otherwise have been considered too risky to treat.” Mark Paul, president of Stryker’s Neurovascular division, added, “The Neuroform Atlas Stent System is the most recent addition to Stryker’s innovative product portfolio, providing a continuum of complete stroke care for patients suffering from cerebrovascular disease. With Neuroform Atlas commercially available in 46 countries, patients around the world are now benefiting from significant advancements in intracranial stents designed specifically for the treatment of wide neck aneurysms. This product is an excellent fit with our mission to make healthcare better.”
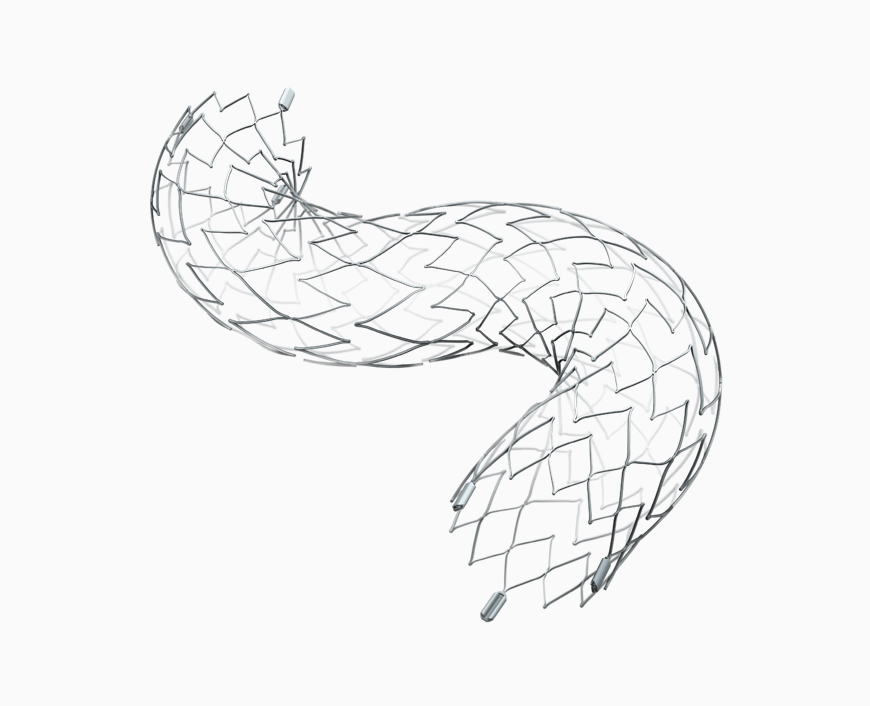
About Neuroform Atlas Stent System
Stryker’s Neuroform Atlas is a small nitinol stent that is used in conjunction with metal coils to pack weakened blood vessel sacs in the brain called aneurysms. Neuroform Atlas is positioned through a small tube and delivered across the neck of the aneurysm to provide support and pack the aneurysm. An estimated 6 million people in the United States have an unruptured brain aneurysm of which roughly 25,000 are treated with endovascular or surgical treatments each year1. Wide neck aneurysms represent less than 10% of unruptured aneurysms treated.
Humanitarian Device. Authorized by federal law for use with neurovascular embolic coils in patients who are ≥ 18 years of age for the treatment of wide neck, intracranial, saccular aneurysms arising from a parent vessel with a diameter of ≥ 2 mm and ≤ 4.5 mm that are not amenable to treatment with surgical clipping. Wide neck aneurysms are defined as having a neck ≥ 4 mm or a dome-to-neck ratio < 2. The effectiveness of this device for this use has not been demonstrated.
Stryker completed enrollment of the anterior arm of the Neuroform Atlas US IDE clinical trial earlier this year while enrollment continues for the posterior arm. This important trial represents the largest data set on adjunctive stent use with intracranial aneurysm coiling and reflects Stryker’s commitment to clinical leadership within the neurovascular space.
About Stryker
Stryker is one of the world’s leading medical technology companies and, together with its customers, is driven to make healthcare better. The company offers innovative products and services in Orthopaedics, Medical and Surgical, and Neurotechnology and Spine that help improve patient and hospital outcomes. More information is available at www.stryker.com.
1. Brain Aneurysm Foundation Statistics. www.bafound.org
Contact(s)
Media contact
Keri Laden
P: 510 413 2534
E: [email protected]